Semiconductors are a group of materials having electrical conductivity intermediate between metals and insulators. It is significant that the conductivity of these materials can be varied over orders of magnitude by changes in temperature, optical excitation, and impurity content. This variability of electrical properties makes the semiconductor materials natural choices for electronic device investigations.
Semiconductor Materials
Semiconductor materials are found in column IV and neighboring columns of the periodic table.
- The column IV semiconductors, silicon and germanium, are called elemental semiconductors because they are composed of single species of atoms.
- In addition to the elemental materials, compounds of column III and column V atoms, as well as certain combinations from II and VI, and from IV, make up the compound semiconductors.
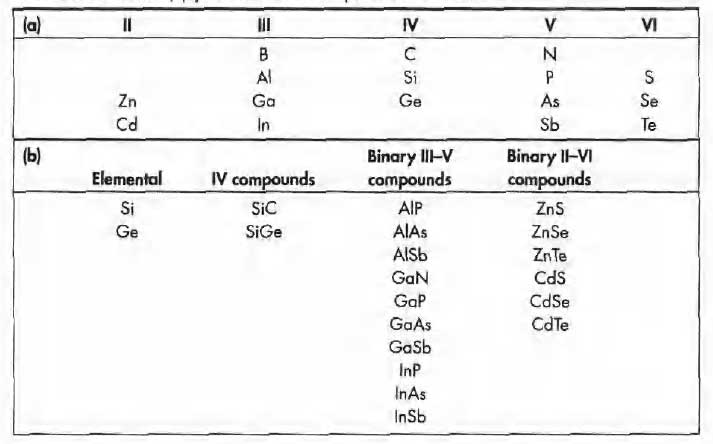
Periodic table - As Table indicates, there are numerous semiconductor materials.
- As the wide variety of electronic and optical properties of these semiconductors provides the device engineer with great flexibility in the design of electronic and optoelectronic functions.
- Silicon is now used for the majority of rectifiers, transistors, and integrated circuits.
- However, the compounds are widely used in high-speed devices and devices requiring the emission or absorption of light.
- The two-element (binary) III-V compounds such as GaN, GaP, and GaAs are common in light-emitting diodes (LEDs).
- As the compounds such as GaAsP and four-element (quaternary) compounds such as InGaAsP can be grown to provide added flexibility in choosing materials properties.
- Fluorescent materials such as those used in television screens usually are II-VI compound semiconductors such as ZnS.
- Light detectors are commonly made with InSb, CdSe, or other compounds such as PbTe and HgCdTe.
- Si and Ge are also widely used as infrared and nuclear radiation detectors.
- An important microwave device, the Gunn diode, is usually made of GaAs or InP.
- Semiconductor lasers are made using GaAs, AlGaAs, and other ternary and quaternary compounds.
- One of the most important characteristics of a semiconductor, which distinguishes it from metals and insulators, is its energy band gap.
- This property, determines among other things the wavelengths of light that can be absorbed or emitted by the semiconductor.
- For example, the band gap of GaAs is about 1.43 electron volts(eV), which corresponds to light wavelengths in the near infrared.
- In contrast GaP has a band gap of about 2.3 eV, corresponding to wavelengths in the green portion of the spectrum.
- The band gap Eg for various semiconductor materials is listed along with other properties in Appendix III.
- As a result of the wide variety of semiconductor band gaps, light-emitting diodes and lasers can be constructed with wavelengths over a broad range of the infrared and visible portions of the spectrum.
- The electronic and optical properties of semiconductor materials are strongly affected by impurities, which may be added in precisely controlled amounts.
- Such impurities are used to vary the conductivities of semiconductors over wide ranges and even to alter the nature of the conduction processes from conduction by negative charge carriers to positive charge carriers.
- For example, an impurity concentration of one part per million can change a sample of Si from a poor conductor to a good conductor of electric current.
