A semiconductor in an extremely pure form is known as an intrinsic semiconductor. The nature of semiconductors is such that even a small amount of certain impurities can change their electrical properties drastically. It is due to this fact, a semiconductor could not be called truly intrinsic, unless the impurity level is very small. For germanium, the impurity level is less than 1 part in 108 parts of Ge and for silicon, it is less than 1 part in 1012 parts of Si. In actual practice, however a semiconductor material with somewhat larger impurity concentrations, then those mentioned above is still called as an intrinsic.
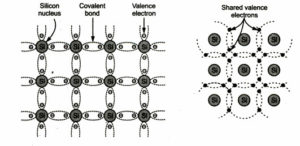
The silicon and germanium are the two most important intrinsic semiconductors. The crystal structure of these materials consist of a regular repetition in 3 dimensions of a unit cell having the form of tetrahedron, with one atom of each vertex. The number of holes generated at a certain temperature equals the number of free electrons.